Benefits of Genetic Expression in Assessing the Efficacy of Bioenergetic Interventions
Written by: Dr. Roberta Kline
One of the biggest challenges in validating the effects of bioenergetic therapies is that the exact mechanisms by which they exert their healing effects are not always specifically known, or we may not know how to measure them objectively.
In this article, we shall review the basics of gene expression and how our genes and epigenetics get translated into health or disease - with a focus on inflammation. This report will also explore how gene expression can provide objective scientific data to show how technologies are interacting with our biology at the deepest level to improve parameters of health and well-being.
DNA contains the genetic code for everything that happens in our biochemistry and biology. Every cell in the body contains the same DNA, and because our DNA is quite long, it is stored in a very compact form in our chromosomes. We normally have 23 pairs of chromosomes.
Specific sections of DNA are called genes, and these genes are discrete areas that contain the codes to make specific proteins. Gene expression is the process of translating this genetic code into specific proteins. These proteins comprise a wide variety of different forms to run our entire biochemistry and metabolism. These include our hormones, receptors, enzymes, transporters, skeletal and heart muscle, and immune system. All of these are used to run various aspects of our biology, from basic functions such as walking, talking, and breathing to more complex processes, including digestion, thinking, and interacting with our environment.
Genes are made into their final proteins through a complex process requiring multiple steps. I’ll simplify the process into the basic steps here. First, the DNA code is transcribed into RNA. That RNA is then edited into a shorter form called messenger RNA, which is translated into a specific amino acid. Multiple amino acids are then assembled into a long chain, which is eventually folded into a three-dimensional protein. The instructions for all of these steps are contained within the DNA.
This one-way process of translating the information in DNA into RNA and then proteins has been termed the Central Dogma of Cellular Biology and has remained unchanged since Francis Crick developed it in 1958. However, recent technological advances have enabled scientists to uncover new discoveries that have expanded our understanding of genetic expression.
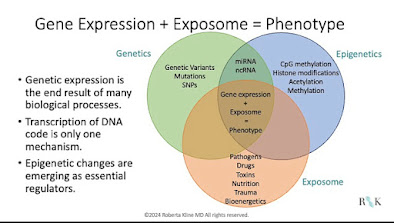
Genetic expression is the end result of multiple biological processes interacting with each other. It turns out that transcription of DNA is only one mechanism that regulates this process. Alterations in the DNA code, including genetic mutations and smaller changes in the DNA called SNPs (single nucleotide polymorphisms), epigenetic changes including some forms of RNA, and the environmental exposures over a person’s lifetime can all impact genetic expression and how it translates into health or disease.
Epigenetics literally means above the genome. These are chemical tags that control access to the DNA that needs to be translated into a protein but do not alter the DNA code itself. Depending on the types and locations of these tags, they can turn genes on or off. Unlike DNA, these chemical tags are reversible, so they can change genetic expression according to the information in the person’s internal and external environment.
This is a crucial mechanism not only for embryological development but also throughout our lifetime. It's the body's way of responding to and adapting to the environment to ensure survival. Some of the earliest and best-known studies have been done with Holocaust survivors [1] and Dutch famine survivors [2], showing changes in epigenetics that helped them adapt to these extreme circumstances. These changes not only occurred in the people who experienced them firsthand, but they have also been passed down to subsequent generations.
But epigenetics is not just for extreme challenges to our survival. We also adapt to our everyday circumstances and environments, and this is part of how we can change our gene expression without changing our genetic code.
There are many types of epigenetic modifications, but the three main ones are DNA methylation, histone modifications, and non-coding RNA. These can either inhibit or activate gene expression, turning genes on and turning genes off as they are needed. While DNA methylation and histone modifications had both been described by the 1970’s [3], DNA methylation is probably the most well-known at this time. It's been the best studied, in large part because it’s technologically easier to assess, and that is what current clinical testing is based on.
We have now expanded beyond DNA to understand that genetic expression is the result of a synergy between our epigenetic regulation and our genetic code, resulting in the ultimate expression—or not—of specific proteins. How these interactions get expressed influences every biological system to determine how our body functions, including whether we are functioning in a state of health or disease.
All of these changes - the genetic code, genomic alterations, and epigenetics can all be inherited so they can all be passed down through the generations. But ultimately the expression of our genes is modifiable, and that’s what’s so powerful when addressing health and disease.
There are multiple multi-directional interactions between the epigenome, genome, and the environment. In addition to the interaction of epigenetics and genetics to influence gene transcription, and the influence of environment on epigenetics, there is yet another mechanism. The end result of genetic expression then provides feedback to all of these systems, creating a feedback loop. It is a very dynamic process that continually operates to adjust, refine, or further adapt genetic expression. While this creates a lot of complexity, it also provides multiple opportunities for both assessment and intervention. One of these that's emerging is the role of PEMF.
While inflammation is a critical system for our survival, ensuring we can heal wounds and fight off infections, it can cause unintended consequences if it is not well regulated. The immune response is designed to provide a robust, short-term response and then go back to its usual surveillance mode. But sometimes it becomes dysregulated and becomes chronic.
Chronic inflammation is central to almost every chronic disease of aging and some chronic diseases that aren't associated with aging. These include heart disease, most if not all types of cancer, neurogenerative diseases including Parkinson's and Alzheimer's, autoimmune diseases, and some of the metabolic diseases such as diabetes and metabolic syndrome. Inflammation plays a role in chronic fatigue syndrome, fibromyalgia, endometriosis, and fibroids, as well as other health issues that you may not directly connect it to including dense breasts, osteoporosis, osteoarthritis, and depression. [4] This is by no means a complete list, but it is clear that chronic inflammation is a significant contributor to many health issues. Finding better ways to address and potentially reverse chronic inflammation can make a huge difference for many people.
The immune system is quite complicated, with many compartments and moving parts that interact with each other to provide a robust defense against invaders for our survival. One key pathway is headed by NF-κB or Nuclear Factor Kappa Beta, and it is considered the master regulator of the immune system.
One reason NF-κB has such wide-ranging effects is that it's known as a nuclear factor. This means that when the NF-κB gene gets activated, the NF-κB protein sets into motion a series of events designed to launch a robust immune response. It does this by attaching to specific locations of the DNA called promoter regions that activate the transcription of other genes. In fact, it initiates the transcription of over 400 genes. These include proinflammatory genes that produce cytokines, chemokines, adhesion molecules, and others. [5]
NF-κB regulates not only a large aspect of the immune system but also many other aspects of cell biology. These include cell proliferation, differentiation, and apoptosis or cell death. It also includes activation of the inflammasome, another component of the immune response, and modulation of the immune system through interaction with the microbiome. [5]
It also includes protection of the mitochondria, an organelle in every cell that is vital for providing the energy for our cells to function. But it doesn’t do this directly. Instead, NF-κB activates another system to protect the mitochondria: the antioxidant system.
NF-κB does not operate in a vacuum. In fact, it is closely intertwined with our antioxidant response in every cell, including in the mitochondria. Activation of the immune response initiates biochemical processes that naturally produce oxidative stress. This is very effective for killing bacteria or viruses that can harm us or facilitating the initiation of healing wounds. Oxidative stress, in turn, can activate the immune response. But too much oxidative stress can damage mitochondria, cell membranes, and even DNA. For this reason, they are elegantly interconnected pathways designed to promote a balanced response that achieves the protection needed without excessive damage.
Oxidative stress will initiate not only activation of the immune response through NF-κB, but will also initiate activation of our antioxidant system. These two systems are connected through two master regulators. NF-κB, which regulates the immune response, and Nrf2, which regulates the endogenous antioxidant response. [6]
When you think of antioxidants, you may think of vitamins such as C or E when you think of the antioxidant response. These are important, particularly in the electron transport chain in the mitochondria, but they provide a limited antioxidant response. Nrf2 initiates a huge antioxidant cascade that is much more powerful than the antioxidant response provided by these vitamins. They're both important, but they're very different.
The Nrf2 protein is encoded by a gene that has a different name, and it’s called NFE2L2, or Nuclear Factor Erythroid-derived 2 Like 2. As its name implies, Nrf2 is also a nuclear factor. When the antioxidant response is initiated, it binds to a specific section of DNA called the antioxidant response element, and this leads to the transcription of several hundred genes downstream. It activates many systems involved in the antioxidant response, including glutathione and thioredoxin production, iron metabolism, and detoxification of endogenous and exogenous toxins. As with NF-κB, Nrf2 also activates genes in other processes, including cell growth, differentiation, and apoptosis, along with energy metabolism. [7]
Mitochondria are often a focus for oxidative stress - and for good reason. They're a main source of endogenous production of free radicals that are the source of oxidative stress. Every time we produce ATP, which is the unit of energy for everything that runs in our body, we are also producing these free radicals. [] It's a normal process, so we also have our built-in antioxidant response to make sure that we don't produce too much oxidative stress and thus damage various compartments or organelles (including the mitochondria themselves) or even our DNA.
The Role of PEMF
Now that we have covered the basics of the biology of the immune and antioxidant responses and some of the key genes that regulate them, let's look at how PEMF may potentially intervene or alter these to create a better state of health.
 Bioenergetics is the study of a flow of energy throughout an organism, and this includes interactions with external and internal environments. As humans, we're very complex and we have a network of different forms of energy that are continually in flux. This is what helps keep us balanced and adapting to everything that goes on in our body and in our environment. Flux is dynamic, and flux in one system or changes in flux in one system will affect other systems. This can result in an imbalance that, over time, can disrupt biochemical and biological systems. [9]
Bioenergetics is the study of a flow of energy throughout an organism, and this includes interactions with external and internal environments. As humans, we're very complex and we have a network of different forms of energy that are continually in flux. This is what helps keep us balanced and adapting to everything that goes on in our body and in our environment. Flux is dynamic, and flux in one system or changes in flux in one system will affect other systems. This can result in an imbalance that, over time, can disrupt biochemical and biological systems. [9]When someone develops a complex or chronic disease, typically these imbalances have been going on for a while. It can be quite challenging to determine what's upstream (or causative) and what's downstream (or secondary effect) of the original imbalance. In order to most effectively target a treatment for a disease, you want to get as close to the initial change in that flux as possible. This is where PEMF is showing great promise.
Calcium Channels
Research has established that the one of the main mechanisms for PEMF exerting its effects is through regulation of calcium influx. It activates the calcium channel and calcium flows into the cell, initiating a cascade of events including transcription of nitric oxide. Nitric oxide then creates a mild oxidative stress as it is a mild prooxidant, and this triggers a cascade of cellular responses. [10]
 One of the challenges in PEMF research currently is the huge variability in outcomes and the often lack of consistency or reproducibility in the research. This has hampered some of the clinical applications in a more rigorous environment. There are several reasons for this. One is that we have found that different cells respond differently. Another factor is that there are many variables in the different technologies and individual protocols.
One of the challenges in PEMF research currently is the huge variability in outcomes and the often lack of consistency or reproducibility in the research. This has hampered some of the clinical applications in a more rigorous environment. There are several reasons for this. One is that we have found that different cells respond differently. Another factor is that there are many variables in the different technologies and individual protocols.  Therefore, lack of consistency between devices, frequencies or intensities used, and duration of treatment are some of the variable elements that can make results confusing, inconsistent, and difficult to compare. What we're now learning is that gene expression may indeed hold the key to changing this.
Therefore, lack of consistency between devices, frequencies or intensities used, and duration of treatment are some of the variable elements that can make results confusing, inconsistent, and difficult to compare. What we're now learning is that gene expression may indeed hold the key to changing this. When Mansourian and Shanei [11] reviewed the literature on PEMF research, they found that only about half of all cells would respond to an intervention. If only half of the cells respond, how can that knowledge be translated into clinical applications where we are working with multiple cell types? This is part of the challenge, and where gene expression is taking a leading role. The authors [11] also documented that the number of experiments using gene expression and related technologies has grown over time, and it is now the biggest category of analysis for experiments.
Gene Expression
Gene expression research has now expanded on our understanding of the impact of PEMF beyond calcium. While the immediate effects of PEMF are related to the calcium influx, we now know that the longer term effects include activation and suppression of various genes, which help to rebalance the system over time. [10]
Thus, PEMF is altering genetic expression. Two of the top genes that are being studied are the genes that we just recently discussed: NF-κB and NFE2L2. This is at least one way that PEMF can impact the immune and antioxidant responses.
 Exploring this connection more closely, it turns out there are additional ways that PEMF is modulating our biology. Research now shows that PEMF also directly stimulates multiple cell signaling pathways. While a discussion of cell signaling is beyond the scope of this article, in essence it is a complex set of pathways that interact with and coordinate between multiple biochemical and biological systems. Recent research in terms of bone repair has documented that the PEMF directly activates multiple cell signaling pathways in osteoblasts, including the inflammatory and antioxidant response. It's also affecting cell cycle, cell growth, apoptosis, mitochondrial function, and biogenesis, [12]
Exploring this connection more closely, it turns out there are additional ways that PEMF is modulating our biology. Research now shows that PEMF also directly stimulates multiple cell signaling pathways. While a discussion of cell signaling is beyond the scope of this article, in essence it is a complex set of pathways that interact with and coordinate between multiple biochemical and biological systems. Recent research in terms of bone repair has documented that the PEMF directly activates multiple cell signaling pathways in osteoblasts, including the inflammatory and antioxidant response. It's also affecting cell cycle, cell growth, apoptosis, mitochondrial function, and biogenesis, [12]This comes as no surprise since we've already seen how the immune system and the antioxidant system are connected to these other biological systems. Therefore, we are greatly expanding our understanding of how PEMF is working to affect both short term and especially long-term changes in our cell biology and in our health.
Two recent studies looked at the impact of PEMF on NF-κB, one related to rheumatoid arthritis and the other to musculoskeletal issues. They both were consistent in their findings that there are direct and indirect effects on the transcription of NF-κB and in downstream genes, and this includes NFE2L2.
In addition to the calcium channel activation mechanism, there is also activation of the RANKL/RANK cell signaling system, which directly downregulates NF-κB. But there are also indirect ways that NF-κB is being downregulated, and these occur in a couple of other cell signaling pathways. These include a pathway involving the adenosine receptors, which decreases the production of prostaglandins and TNF- alpha, which is a primary trigger of NF-κB. Another is activation of the TRK cell signaling pathway, which activates mTOR and results in downregulation of NF-κB. [13, 14] This involvement of multiple cell signaling pathways by PEMF results in a decrease in NF-κB and increase in NFE2L2, both of which contribute to lowering inflammation and oxidative stress.
In referring to the literature review by Mansourian and Shanei [11], two different studies that they looked at evaluated varying amounts of frequencies and intensities in terms of PEMF treatment of cells. Their results showed that by altering any one of these variables, gene expression could potentially be changed – and thus, potentially the outcomes. This variability in outcomes is one of the key challenges that PEMF research needs to address, and gene expression is a very elegant way to evaluate this. Therefore, as we expand PEMF research, we need to include gene expression as it will help to evaluate multiple different PEMF regimens and will give us a much more in-depth view of how multiple genes and multiple pathways are being impacted with each.
Single Exposure Study
Here is an example of a recent study that looked at the effects of a single exposure on gene expression.
Panja et al [15] simplified the variables by looking at only one dose and one exposure. In addition to evaluating gene expression related to inflammation and oxidative stress responses, they also measured gene expression across multiple different systems that we have covered in this article, including cell growth, differentiation, and apoptosis.
These researchers used human intestinal epithelial cells for their cell culture studies. The use of an in vitro technique is consistent with most research that has been done with PEMF in terms of gene expression. First, they obtained a baseline expression measuring over 60,000 genes and how they were being expressed. Then they inoculated the cells with lipopolysaccharide, which is a bacterial endotoxin with a known inflammatory response profile, followed by another assessment to evaluate what happened to the gene expression after they in introduced this pro-inflammatory element. Gene expression was then reassessed again after a single exposure of PEMF.
Fourteen hours later they again measured gene expression. The researchers chose 14 hours because they wanted to capture both the immediate response and the downstream effects that would take a little longer to show up.
What was interesting was that their initial results were somewhat surprising. They looked at more than 60,000 genes and how they were expressed, and noticed that the total number of genes being expressed didn't significantly change before and after treatment with the lipopolysaccharide or with PEMF. So, they dug deeper.
What they discovered is that while the total number of genes being expressed didn't change, which genes and how they were being expressed or suppressed did. Specifically related to inflammation-associated genes, they found that PEMF restored normal gene expression in these multiple genes associated with inflammation that had been upregulated by the lipopolysaccharide. PEMF also reversed the downregulation that had occurred in the genes responsible for regulating the inflammatory response. In both cases, they found that PEMF restored a normal balance between the expression of pro-inflammatory and anti-inflammatory genes, enabling a return to normal functioning.
In addition to reporting on inflammation in this paper, they also shared that they looked at PEMF and gene expression in other functional groups. These included multiple other systems that were not specifically reported on in this paper but will hopefully be published in a subsequent paper soon. With all of this data, it’s illustrating the concept that we are altering flux in one system and there are multiple downstream effects because these systems are interconnected.
Future Directions
Single-cell research has been absolutely powerful, enabling understanding at a very detailed level of how PEMF can alter gene expression. One of the challenges is that, as human beings, we are not single cells. We are a very complex compilation of multiple different types of cells in different states of health and function (or flux) that are all communicating with each other and interacting with the environment.
The logical next question to address is how to translate the single-cell research into clinical practice. This is where gene expression is well-positioned to look at how these multiple cells are operating within multiple systems as part of a whole human being and responding to PEMF treatment. We can do this using the same technologies as used with single-cell experiments, and the advancements in our ability to interpret large amounts of data now gives us this opportunity that even a few years ago was very limited.
 We know we can measure gene expression changes before and after PEMF treatment. We can then correlate these with known clinical parameters or tests that are non-invasive, and also with subjective measurements of patient symptoms. Additional questions help us go even deeper. Do the subjective measurements correlate with the objective data? Is there a difference between targeted treatment to a specific location versus whole body treatment?
We know we can measure gene expression changes before and after PEMF treatment. We can then correlate these with known clinical parameters or tests that are non-invasive, and also with subjective measurements of patient symptoms. Additional questions help us go even deeper. Do the subjective measurements correlate with the objective data? Is there a difference between targeted treatment to a specific location versus whole body treatment? Many of these inflammatory diseases are systemic, so finding the answers to these questions will help us refine and really understand the impact systemically of PEMF, even if it's done locally. Conversely, we may learn that in some cases it may be more effective to treat a broader area.
These are all questions that gene expression research can help us answer fairly quickly. Referring back to our earlier discussion of many of the factors that influence gene expression, there are also a lot of other interventions that we can do with diet and lifestyle and potentially other kinds of therapies. How do these all interact? Can we make PEMF even more powerful or conversely, can PEMF potentiate some of these other interventions?
These questions are ripe for exploration now that we have the availability of gene expression research and interpretation.
References:
1) Yehuda, R. et al. (2005). Influences of maternal and paternal PTSD on epigenetic regulation of the glucoticoid receptor gene on Holocaust survivor offspring. American Journal of Psychiatry, 173, 872-880.
2) Kennedy, B. K., Berger, S. L., Brunet, A., Campisi, J., Cuervo, A. M., Epel, E. S., … & Rando, T. A. (2014). Geroscience: Linking aging to chronic disease. Cell, 159(4), 709-713.
3) Holliday R, Pugh J. DNA modification mechanisms and gene activity during development. Science 1975;187(4173):227-232.
4) Jogpal, V., Sanduja, M., Dutt, R. et al. Advancement of nanomedicines in chronic inflammatory disorders. Inflammopharmacol 30, 355–368 (2022).
5) Zhang, S., Paul, S., & Kundu, P. (2022). NF-κB Regulation by Gut Microbiota Decides Homeostasis or Disease Outcome During Ageing. Frontiers in Cell and Developmental Biology, 10
6) Zoja, C., Benigni, A., & Remuzzi, G. (2014). The Nrf2 pathway in the progression of renal disease. Nephrology Dialysis Transplantation, 29(suppl_1), i19-i24
7) Tonelli C, Chio IIC, Tuveson DA. Transcriptional Regulation by Nrf2. Antioxidants & Redox Signaling. Dec 2018.1727-1745.
8) García-García, F. J., Monistrol-Mula, A., Cardellach, F., & Garrabou, G. (2020). Nutrition, Bioenergetics, and Metabolic Syndrome. Nutrients, 12(9).
9) Swerdlow, R. H. (2014). Bioenergetic medicine. British Journal of Pharmacology, 171(8), 1854-1869; Richard Gerber, Vibrational Medicine: New Choices for Healing Ourselves, Bear & Co., 1988 & 2000.
10) Luigi C, Tiziano P (2020) Mechanisms of Action And Effects of Pulsed Electromagnetic Fields (PEMF) in Medicine. J Med Res Surg 1(6): pp. 1-4.
11) Mahsa Mansourian, Ahmad Shanei. Evaluation of Pulsed Electromagnetic Field Effects: A Systematic Review and Meta-Analysis on Highlights of Two Decades of Research In Vitro Studies. BioMed Research International, 2021.
12) Wang, A., Ma, X., Bian, J., Jiao, Z., Zhu, Q., Wang, P., & Zhao, Y. (2024). Signalling pathways underlying pulsed electromagnetic fields in bone repair. Frontiers in Bioengineering and Biotechnology, 12, 1333566.
13) Hu, H., Yang, W., Zeng, Q., Chen, W., Zhu, Y., Liu, W., Wang, S., Wang, B., Shao, Z., & Zhang, Y. (2020). Promising application of Pulsed Electromagnetic Fields (PEMFs) in musculoskeletal disorders. Biomedicine & Pharmacotherapy, 131, 110767.
14) Ross, CL, Ang, DC, Almeida-Porada, GA. Targeting Mesenchymal Stromal Cells/Pericytes (MSCs) With Pulsed Electromagnetic Field (PEMF) Has the Potential to Treat Rheumatoid Arthritis. Front. Immunol., 2019 Vol 10.
15) Panja A, Binder R, Binder S. Focused-pulsed electromagnetic field treatment reverses lipopolysaccharide-induced alterations in gene expression profile in human gastrointestinal epithelial cells. Int J Health Allied Sci [serial online] 2021 [cited 2024 Mar 14];10:55-69.